Introduction
The ThinkCancer! Study builds on an earlier piece of work – the WICKED (Wales Interventions and Cancer Knowledge about Early Diagnosis) trial which involved consulting with GP Practices throughout Wales to develop and test a series of workshops for general practice teams to improve cancer diagnosis and referral rates.
During the ThinkCancer! workshops our aim is to provide GPs and other clinical staff with updates on the latest changes to clinical guidelines and support to develop and enhance their own knowledge and skills regarding vague and ‘harder to spot’ symptoms of cancer. We work with reception teams to increase their general awareness of potential cancer symptoms and then help the whole practice to develop their own ‘Cancer Safety-Netting Plan’.
GP Practices taking part in the trial will be randomly allocated to those that have the workshops (the intervention group) and those that do not have them (the control group) to see if the ThinkCancer! workshops do help improve cancer diagnosis.
In order to assess the impact of the research study we will be reviewing anonymised data from patient records from GP practices that are taking part in the study. This will only involve patients who have recently been referred on for further tests by their GP with possible signs of cancer and will be limited to information related to the possible cancer diagnosis. We will not look at any other unrelated data.
The patient experience
Some patients may be contacted to ask if they would be willing to talk about their experiences of having tests done to investigate a possible cancer – this is completely voluntary. Patient data would only ever be seen by healthcare professionals and researchers who have signed confidentiality agreements with the NHS and all data is anonymised before leaving the GP practice.
If the GP practice is taking part in the ThinkCancer! trial patients will be contacted and given further details which also include information on how to opt out if they do not wish to take part in the study. Patients can opt out without having to give any reason and this would not affect their usual care or relationship with their GP practice. There will also be a poster in GP practices advising that the surgery is taking part in the trial with further information.
What we hope to achieve in this trial
We want to see whether the ThinkCancer! Workshops, designed to help all staff in GP practices, help to reduce the time it takes to diagnose cancers and that the workshops would be an effective use of resources if rolled out across the wider NHS.
Further information is available on the website including some experiences from patients that have taken part in the interview process from the earlier study.
Development of ThinkCancer!
In comparison with other Western countries, Wales has relatively poor cancer outcomes (The Lancet 2019) with late diagnosis being a major factor (CPGC, 2020). Evidence from the International Cancer Benchmarking Partnership has shown that GPs in Wales are less likely to refer or investigate (Rose et al., 2015) and that adherence to cancer guidelines is lower (Nicholson, Mant, Neal et al., 2016). This has a negative effect on cancer outcomes.
These deficiencies have been recognised by the Welsh NHS who earlier this year published details of a three-year Cancer Improvement Plan (Wales Cancer Network 2023), which identifies a series of measures that are being undertaken to improve the experience and outcomes for people affected by cancer. In this it is recognised that not all the deficiencies are the fault of primary care but are systemic in nature and will require additional investment and improvement in digital systems and access. However, primary care has an exceptionally important role in the early detection and treatment of cancer - around 60% of cancers are diagnosed through primary care (Hanna 2020), and almost half (49%) of avoidable delays occur within primary care (Swann 2020).
‘ThinkCancer!’ tackles this problem by using workshops to provide GPs, associated healthcare professionals and other non-clinical staff with an update on the latest changes to clinical guidelines and to develop and enhance their own knowledge and skills regarding vague and ‘harder to spot’ symptoms of cancer. It is hoped that by doing this cancer outcomes can be improved.
Background to the ThinkCancer! Trial
The ThinkCancer! trial builds upon an earlier programme of research called Wales Interventions and Cancer Knowledge about Early Diagnosis (WICKED). The overall aims of the WICKED programme were to improve the quality and consistency of primary care approaches to improve timely diagnosis of cancer. General practitioners in Wales contributed to every step of this programme of research.
The WICKED programme was developed into work packages one to five where a variety of methods were used to collect and integrate data about cancer diagnosis in primary care to produce an intervention which would form the basis of the ThinkCancer! Trial.
Work Packages 1 to 3
Work Packages 1 and 2 involved producing an online survey including a Discrete Choice Experiment (DCE) sent to all GPs in Wales. There were then twenty telephone interviews with GPs followed by focus groups held with four practice teams.
Throughout this process various stakeholder groups were consulted, and behaviour change theory underpinned the work allowing a target behaviour to be agreed culminating in the completion of Work Package 3 - GPs thinking of and acting on clinical presentations* that could be cancer (The ThinkCancer! intervention).
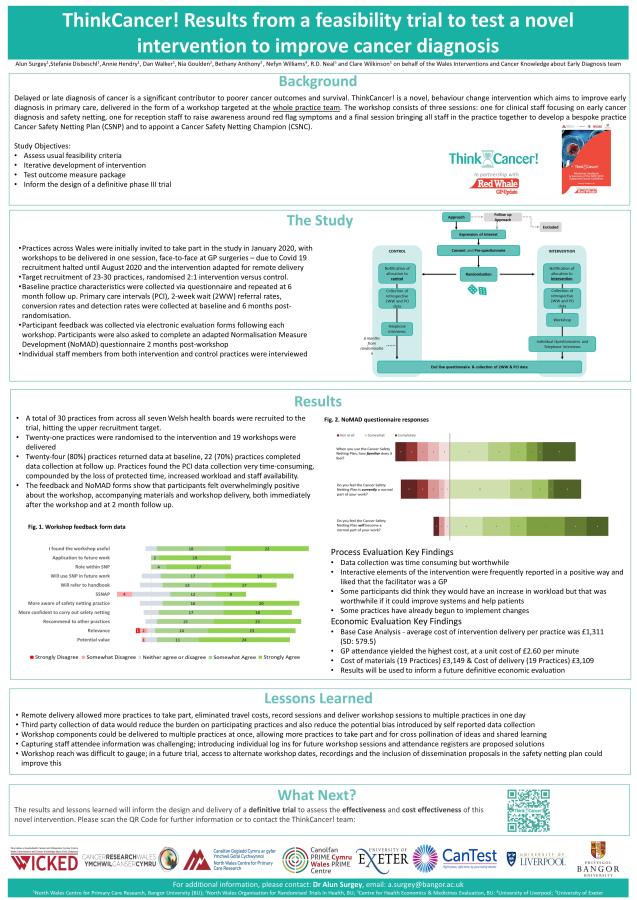
Work Package 4 - The Feasibility Study
This intervention was tested on a small scale in the ThinkCancer! Feasibility Study. This helped evaluate the design of a larger Phase III study to determine whether ThinkCancer! can reduce referral times and whether it is cost effective. The feasibility study was delivered successfully and the whole-practice workshop series was clearly timely and much appreciated by general practices. The recruitment target was reached, and progression criteria indicated that a phase III trial is feasible and acceptable.
Work Package 5 – Preparation for Phase 3 trial
Lessons learnt during the feasibility study led to further refinement of the ThinkCancer! intervention in Work Package 5. Several methodological issues which were highlighted have been addressed in the phase III trial and adaptations have been made. These have further been informed by PPI input, context changes in primary care (example Continuing Professional Development (CPD) training, and comments following a peer review process.
More information about the WICKED programme can be found here WICKED.bangor.ac.uk
Case Note Review
Diagnosing cancers earlier improves outcomes for patients, and as earlier treatment tends to be less intensive it also saves the NHS money. The United Kingdom’s five-year cancer survival rates are worse than other high-income countries (CRUK, 2017, Arnold 2019) and delays in cancer diagnosis and starting treatment are part of the reason for this (Hanna 2020).
Most patients diagnosed with a cancer in the UK have been seen in General Practice with symptoms that may be related to their cancer (Swann 2018) and general practice is where almost half of avoidable delays in cancer diagnosis happen (Swann 2020).
As part of a the larger ThinkCancer!’ trial, we plan a further study to examine in-detail the cases of 200-300 patients who have waited much longer than the average for their cancer type to be diagnosed. Currently little is known about the reasons why some patients are diagnoses much later than other who have the same kind of cancer, but our aim is to explore the reasons behind this. We hope that this will help to develop ways of preventing other patient from experiencing similar delays in the future.
Patient and Public Involvement (PPI)
Janice Rose, ThinkCancer! PPI Representative
Janice Rose has been involved throughout the WICKED programme and is a key member of the ThinkCancer! team, representing the public and patients and ensuring their voice is heard throughout the process.
“I got involved with researchers working in the field of cancer after my breast cancer diagnosis and my mum’s diagnosis of Cancer of Unknown Primary (CUP). Both happened in 2009. Since then I have worked locally, regionally and nationally with researchers representing the patient perspective in various research studies taking place and generally helping research into cancer where I can.”
“My link to the Wales Interventions and Cancer Knowledge about Early Diagnosis (WICKED) Programme came through my link to the NCRI Primary Care Group. I have worked with the researchers since the study started.”
“I am pleased to be one of the patients working with the research team looking at ways to help doctors and other staff in GP practices in Wales to help improve early cancer diagnosis for their patients. The project is very worthwhile as early diagnosis of cancer is so important for patients in terms of the outcomes after their diagnosis.”
“Having patients working with the research team allows for the voice of the patient to be listened to and considered throughout the research project. It is very important.”
Elfyn Jones, ThinkCancer! PPI Representative
Elfyn Jones is a new recruit to the ThinkCancer! PPI team and has quickly become a highly valued member of the team, representing the public and patients, and ensuring their voice is heard throughout the process.
“A semi-retired 60 yr old survivor of advanced Seminoma (testicular cancer), I live in a small village close to the base of Yr Wyddfa (Snowdon) on the edge of Eryri National Park. Am an avid outdoor person, a very keen mountaineer and rock climber having climbed all over the world. I’m also a coordinator and active member of Llanberis Mountain Rescue Team, as well as being the owner of a 6yr old hyperactive spaniel called Hamish!”
“My cancer journey was quite convoluted – a late diagnosis that took over twelve months to be confirmed, despite several scans, biopsies and even surgery. Thankfully once diagnosed, the treatment, while gruelling and intense, appears to have been very successful, and 8 yrs down the line, there appears to be no sign of a reoccurrence”
“I’m involved in this project, as I know from my own experience, especially in the early stages, that some obvious symptoms and signs can be missed, even by the most diligent professionals (who are inevitably overworked and under pressure), and that this is especially so in otherwise fit and healthy individuals. and that early diagnosis makes a huge difference to both outcomes and the treatment options”
Professor Clare Wilkinson
Co-Chief Investigator & Professor of General Practice & Co-Chief Investigator
c.wilkinson@bangor.ac.uk
Professor Richard Neal
Co-Chief Investigator & Professor of Oncology & Professor of General Practice
R.D.Neal@leeds.ac.uk
Nic Nikolic
Trial Manager
n.nikolic@bangor.ac.uk
Dr Dan Walker
GP Educator
d.walker@bangor.ac.uk
Dr Kausthubha Raman
Academic Clinical Fellow
k.raman@bangor.ac.uk
Dr Annie Hendry
Qualitative Researcher
a.hendry@bangor.ac.uk
Clio Evans
Research Project Support Officer (Qualitative)
Clio.evans@bangor.ac.uk
Benji Cotterell
Research Project Support Officer
b.cotterell@bangor.ac.uk
Stefanie Disbeschl
Research Project Support Officer
stefanie.disbeschl@bangor.ac.uk
Richard Evans
Senior Research Administrator
Richard.evans@bangor.ac.uk
Emma Jones
Clerical Officer
Emma.Jones@bangor.ac.uk
Dr Kirstie Pye
Clinical Trials Unit Manager
k.pye@bangor.ac.uk
Dr Nia Goulden
Trial Statistician
n.goulden@bangor.ac.uk
Dr Rachel Evans
Senior Trial Statistician
r.evans@bangor.ac.uk
Huw Williams
CT Digital Systems Officer
huw.w@bangor.ac.uk
Thomas Goff
CT Data Systems Officer
t.goff@bangor.ac.uk
Casey Nolan
Trial IT Delivery Facilitator
casey.nolan@bangor.ac.uk
Bethan Lewis
Quality Assurance Support Officer
bethan.lewis@bangor.ac.uk
Karolina Rusiak
Quality Assurance Officer
k.rusiak@bangor.ac.uk
Elizabeth Denis
Quality Assurance Support Officer
e.denis@bangor.ac.uk
Professor Rhiannon Tudor Edwards
Professor of Health Economics & Health Economic Oversight
r.t.edwards@bangor.ac.uk
Victory Ezeofor
Statistical Modeller (Health Economics)
v.ezeofor@bangor.ac.uk
Dr Bethany Anthony
Research Officer (Health Economics)
b.anthony@bangor.ac.uk
Professor Nefyn Williams
Professor of Primary Care / Collaborator
University of Liverpool
Professor Kate Brain
Professor of Health Psychology / Collaborator
Cardiff University
Professor Andrew Carson-Stevens
Professor of Patient Safety / Collaborator
Cardiff University
Janice Rose
PPI Representative & Co-applicant
Elfyn Jones
PPI Representative
Keith Holt
PPI Representative
Helen Rees
PPI Representative
Sian Rogers
PPI Representative

We would like to extend our thanks to our funders Cancer Research Wales and North West Cancer Research, and to the members of the public who support these charities, without whom our work would not be possible.
ThinkCancer! Newsletters
September 2025

SAPC 2023 - Title: The ThinkCancer! Intervention: results and lessons learned from a phase II feasibility trial in Wales.
CREST 2023 (Presented by Dr Dan Walker & Dr Annie Hendry) - Title: ThinkCancer!: A pragmatic randomised controlled phase III trial of a novel behavioural intervention for primary care teams to promote earlier cancer diagnosis
HCRW 2023 (Presented by Clio Evans) - Title: Weaving Patient and Public Voices into the ThinkCancer! Study
PRIME 2023 (Presented by Clio Evans) - Title: Innovating Efficient Ways of Acquiring Patient and Public Feedback for the ThinkCancer! Study
CaPRI 2023 (Presented by Stefanie Disbeschl) - Title: The ThinkCancer! Intervention: results and lessons learned from a phase II feasibility trial in Wales
SW SAPC 2024 (Presented by Clio Evans) - Title: Weaving Patient and Public Voices into the ThinkCancer! Study
SAPC 2024 (Presented by Clio Evans) - Title: Creating robust safety netting systems to expedite cancer diagnosis: The progress of a phase III RCT
SAPC 2024 (Presented by Dr Annie Hendry) - Title: How can the whole practice approach to training and education foster a healthy system in facilitating early cancer diagnosis in primary care? Qualitative findings from a feasibility RCT.
SAPC North 2024 (Presented by Benji Cotterell) - Title: The importance of Patient and Public Involvement and Patient Advisory Groups on a phase III clinical trial.
PRIME 2024 (Presented by Clio Evans) - Devising Solutions to facilitate General Practices' participation in the ThinkCancer! Randomised Controlled Trial.
PRIME 2024 (Presented by Benji Cotterell) - The importance of Patient and Public Involvement and Patient Advisory Groups on a phase III clinical trial.
SAPC 2025 (Presented by Clio Evans) - An in-depth exploration of key stakeholder perspectives to establish context for implementing ThinkCancer!, a novel behaviour intervention.
SAPC 2025 (Presented by Benji Cotterell) - Reaching over 1 million patients and accessing patient records via VPNs in primary care.
Ca-PRI 2025 (Presented by Clio Evans) - Setting the context for ThinkCancer!: A qualitative exploration of stakeholder perspectives.
NWCR Symposium 2025 (Presented by Stefanie Disbeschl) – PhD presentation.
ThinkCancer! Supports “Stripe a Pose” for World Cancer Research Day
The ThinkCancer! team were delighted to support Cancer Research Wales’ “Stripe a Pose” campaign for World Cancer Research Day, proudly showing our stripes to champion life-saving cancer research across Wales. Through a series of team photos and media posts, we helped shine a light on the crucial role of research in advancing early diagnosis and cancer prevention. The campaign was an important opportunity for us to stand alongside colleagues and communities across Wales, united in our shared commitment to improving cancer outcomes for all.



Cancer: Doctors urged by charity to identify symptoms sooner
Cancer: Doctors urged by charity to identify symptoms sooner - BBC News
Meet the Researcher: Clio Evans
SW SAPC 2024 & HCRW Conference 2023

WONCA 2023
Read more about the WICKED Programme and the previous Work Packages by clicking here to read our publication in the BJGP.
*clinical presentations: symptoms, clinical signs and the context (e.g. past medical history, risk factors, previous consultations) during a primary care consultation.
For more information about this study, please contact the study team on thinkcancer@bangor.ac.uk
© Bangor University
53.04758708462477, -3.0139847228667436





